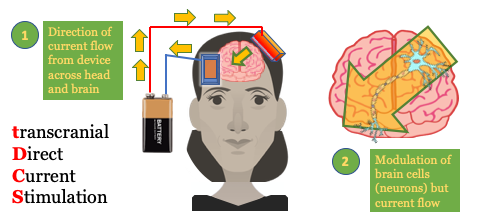
Transcranial Direct-Current Stimulation (tDCS) is a portable, wearable brain stimulation technique that delivers a low electric current to the scalp. A fixed current between 1 and 2 mA is typically applied 1 . tDCS works by applying a positive (anodal) or negative (cathodal) current via electrodes to an area. tDCS is a neuromodulation technique that produces immediate and lasting changes in brain function. The position of the anode and cathode electrodes on the head is used to set how current flows to specific brain regions. The current delivered by tDCS is NOT strong enough to trigger an action potential in a neuron; instead its “sub-threshold” changes the pattern of already activity neurons. Think of the brain as active, trying to do or learn something, and tDCS coming along to boost this ongoing activity. At the cellular level 2 , tDCS changes neuronal firing and by strengthening synaptic transmission between neurons by augmenting synaptic plasticity 3 which is, in turn, the cellular basis of learning. tDCS is often combined with training. Training in itself produces learning (synaptic plasticity), and concurrent tDCS amplifies these effects (enhances synaptic plasticity). Some clinical applications tDCS is currently being explored for are depression, schizophrenia, aphasia, addiction 4 , epilepsy, chronic pain (migraine, fibromyalgia), attention, and motor rehabilitation. tDCS is also used for non-medical wellness applications, for example accelerated learning 5 , focus, relaxation, and meditation. 6

tDCS devices are small battery powered devices. There is usually a control panel that allows you to program the device (to set the duration and intensity of stimulation). Electrodes are placed on the head and held in place by headgear — usually an elastic strap. A cable connects each electrode to the stimulator. When the stimulator is turned on, current flows from the device to the electrode, and subsequently through the brain. Professional grade stimulators have many features that help ensure stimulation is tolerable and reliable. This includes an impedance meter and a current meter.
Research on the side-effects of tDCS is ongoing, but so far the established side-effects are minor 7 , and restricted to the electrode location. They include temporary skin redness, itching, and tingling. Other suggested side-effects of tDCS include headache, nausea, and dizziness. It should be noted that these latter three side-effects have been illustrated to occur at nearly the same rate as sham stimulation (fake stimulation) 8 When tDCS is applied inadequately, other side-effects can occur such as a phosphene which is a temporary, non-dangerous flash of light. This can occur if electrodes are placed too close to the eye. Additionally, incorrect tDCS administration can elicit standard skin burns. There is no scientific evidence that demonstrates lasting injury or irreversible side-effects from tDCS. Nonetheless it should be noted that all of the tolerability and safety data on tDCS comes from controlled human trials using specialized equipment and strictly controlled protocols (e.g., limiting current duration, number of sessions).
During tDCS, most people will feel a mild tingling, prickling, itching, or warmth. These sensations are not painful and go away when stimulation stops. However the “tolerability” of tDCS depends on the quality of accessories, set-up procedures, and using an intensity (few mA) and duration (tens of minutes) consistent with tDCS standards. 23
While questions remain about the best applications for tDCS, there are decades of research denoting its implicated mechanism. Recent work suggests glial activation and the alteration of intracellular cAMP and calcium concentrations to largely contribute to tDCS’s effects 9 It is also understood that the plasticity of the human brain can allow lasting excitability changes as a result of tDCS application, namely, long-term potentiation (LTP) and long-term depression (LTD).
In the United States tDCS has the regulatory status of “investigational” 10 . This gives no indication of efficacy; it means the FDA has not issued an opinion. Typically, The FDA does not issue an opinion until companies show interest in marketing a device. In the United States, companies are not allowed to market tDCS for a clinical indication such as “treatment of depression” or “treatment of epilepsy.” Doctors in the United States are allowed to provide “off-label” treatment, that is, treatment that is not approved by the FDA for the given indication. Research centers worldwide are allowed to test tDCS in controlled clinical trials. In such trials every subject must sign an informed consent sheet. You can find a listing of tDCS trials here: clinicaltrials.gov. In the EU, tDCS is approved for the treatment of pain and depression. You can use the free tool below to find doctors and clinics who provide tDCS based treatment
tDCS is currently not FDA approved. That means that the USA FDA has not evaluated and approved a “marketing” application from a company. It does not mean the USA FDA has made a formal decision on the efficacy or safety of tDCS for any specification induction such as Depression or Pain. In the USA tDCS for medical use is considered “Investigational”. The FDA does not read clinical trials and makes decisions based on the literature, the USA FDA only respond to “marketing” requests made by specific companies. The FDA typically does not regulate non-medical use of devices, which includes uses for “wellness” purposes 11 . In this sense, it is important to note tDCS is broadly considered by researchers and experts as considered low-risk. In fact, the FDA has provided “513g” letters to several companies explicitly allowing them to market tDCS for specific non-medical uses. The FDA also does not regulate the practice of medicine, meaning they don't regulate doctors. For this reason many doctors provide treatments that are “off-label” - things that doctors think work but do not have a “marketing” label from the FDA to the company. tDCS is approved for medical treatment in much of the world 12 including the European Union 13 , Israel, and Singapore. In summary while tDCS is currently not approved by the FDA, this does not mean tDCS can not be legally tested or used in specific contexts.
The cost of tDCS devices can vary from around $100 for a basic “consumer” tDCS devices to thousands of dollars for “research-grade” tDCS systems. There are a broad range of features and capabilities across different devices.
The anxiety reduction effects of tDCS have been reported in several clinical trials 14 from top medical center. In the USA, tDCS is not approved for treatment of medical anxiety. Techniques related to tDCS, such as transcranial Alternating Current Stimulation (tACS) have shown promise for anxiety in clinical trials 15 . Another related technique Cranial Electrotherapy Stimulation (CES) is FDA cleared for Anxiety 16 .
Several clinical trials have reported tDCS can treat depression 17 . tDCS also has much less side effects than drugs 18 . In the USA, tDCS is not approved for the treatment of depression. In much of the world, including across Europe, tDCS is approved for treatment of depression 19 .
tDCS is used for many different applications that involve changing the brain to influence how people think or feel 20 . tDCS is often combined with some other form of activity or training, with the goal of tDCS boosting that specific brain activity. tDCS has been shown to make people learn faster 21 . For example, tDCS can enhance mindfulness (E-meditation). People are also interested in tDCS to boost 22 “working memory”.
The Content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition.
References1. Woods A.J., Antal A., Bikson M., Boggio P.S., Brunoni A.R., Celnik P.…Nitsche M.A. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clinical Neurophysiology. 2016;127(2):1031–1048. 2. Physiological Basis of tDCS talk by Dr. Michael Nitsche on youtube 3. Kronberg, G., Rahman, A., Sharma, M., Bikson, M. and Parra, L., 2020. Direct Current Stimulation Boosts Hebbian Plasticity In Vitro. Brain Stimulation 4. Ekhtiari H, Tavakoli H, Addolorato G, Baeken C, Bonci A, Campanella S, Castelo-Branco L, Challet-Bouju G, Clark VP, Claus E, Dannon PN, Del Felice A, den Uyl T, Diana M, di Giannantonio M, Fedota JR, Fitzgerald P, Gallimberti L, Grall-Bronnec M, Herremans SC, Herrmann MJ, Jamil A, Khedr E, Kouimtsidis C, Kozak K, Krupitsky E, Lamm C, Lechner WV, Madeo G, Malmir N, Martinotti G, McDonald W, Montemitro C, Nakamura-Palacios EM, Nasehi M, Noël X, Nosratabadi M, Paulus M, Pettorruso M, Pradhan B, Praharaj SK, Rafferty H, Sahlem G, Salmeron BJ, Sauvaget A, Schluter RS, Sergiou C, Shahbabaie A, Sheffer C, Spagnolo PA, Steele VR, Yuan T-F, van Dongen J, Van Waes V, Venkatasubramanian G, VerdejoGarcía A, Verveer I, Welsh J, Wesley MJ, Witkiewitz K, Yavari F, Zarrindast M-R, Zawertailo L, Zhang X, Cha Y-H, George TP, Frohlich F, Goudriaan AE, Fecteau S, Daughters SB, Stein EA, Fregni F, Nitsche MA, Zangen A, Bikson M, Hanlon CA (2019). Transcranial Electrical and Magnetic Stimulation (tES and TMS) for Addiction Medicine: A consensus paper on the present state of the science and the road ahead. Neuroscience & Biobehavioral Reviews. 2019. 104: 118-140 5. Coffman, B., Trumbo, M., Flores, R., Garcia, C., van der Merwe, A., Wassermann, E., Weisend, M. and Clark, V., 2020. Impact Of Tdcs On Performance And Learning Of Target Detection: Interaction With Stimulus Characteristics And Experimental Design. 6. E-meditation: A new tool for an ancient technique on Medical X Press by Medical University of South Carolina 7. Bikson M., Grossman P., Thomas C., Zannou A. L., Jiang J., Adnan T., et al. (2016). Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul. 9 641–661. 10.1016/j.brs.2017.07.001 8. Brunoni, A. R., Amadera, J., Berbel, B., Volz, M. S., Rizzerio, B. G., & Fregni, F. (2011). A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation.International Journal of Neuropsychopharmacology, 14(8), 1133-1145. 9. Monai, H., Ohkura, M., Tanaka, M., Oe, Y., Konno, A., Hirai, H., … & Hirase, H. (2016). Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nature communications, 7. 10. "Off-Label" and Investigational Use Of Marketed Drugs, Biologics, and Medical Devices - Guidance for Institutional Review Boards and Clinical Investigators fda.gov 11. General Wellness: Policy for Low Risk Devices - Guidance for Industry and Food and Drug Administration Staff fda.ggOyR0iXCbMQv3Xipma34MD 12. Fregni F, Nitsche MA, Loo CK, Brunoni AR, Marangolo P, Leite J, Carvalho S, Bolognini N, Caumo W, Paik NJ, Simis M, Ueda K, Ekhtiari H, Luu P, Tucker DM, Tyler WJ, Brunelin J, Datta A, Juan CH, Venkatasubramanian G, Boggio PM, Bikson M. Regulatory Considerations for the Clinical and Research Use of Transcranial Direct Current Stimulation (tDCS): review and recommendations from an expert panel. Clin Res Regul Aff. 2015 Mar 1;32(1):22-35. 13. Soterix Medical, Inc. Receives CE Mark Approval for 1x1 tDCS Depression Therapy | soterixmedical.com 14. Nishida K, Koshikawa Y, Morishima Y, et al. Pre-stimulus brain activity is associated with state-anxiety changes during single-session transcranial direct current stimulation. Front Hum Neurosci. 2019;13:266. doi:10.3389/fnhum.2019.00266 15. Clancy KJ, et al. Lasting connectivity increase and anxiety reduction via transcranial alternating current stimulation. Soc. Cogn. Affect. Neurosci. 2018;13:1305–1316. 16. Neurological Devices; Reclassification of Cranial Electrotherapy Stimulator Devices Intended To Treat Anxiety and/or Insomnia; Effective Date of Requirement for Premarket Approval for Cranial Electrotherapy Stimulator Devices Intended To Treat Depression | federalregister.gov 17. Coffman, B., Trumbo, M., Flores, R., Garcia, C., van der Merwe, A., Wassermann, E., Weisend, M. and Clark, V., 2020. Impact Of Tdcs On Performance And Learning Of Target Detection: Interaction With Stimulus Characteristics And Experimental Design. 18. Andre R. Brunoni, M.D., Ph.D., Adriano H. Moffa, Psy.D., Bernardo Sampaio-Junior, M.D., Lucas Borrione, M.D., Marina L. Moreno, Psy.D., Raquel A. Fernandes, Psy.D., Beatriz P. Veronezi, Psy.D., Barbara S. Nogueira, Psy.D., Luana V.M. Aparicio, M.D., Lais B. Razza, Psy.D., Renan Chamorro, Psy.D., Luara C. Tort. Trial of Electrical Direct-Current Therapy versus Escitalopram for Depression List of authors. 19. Soterix Medical Wins CE Mark for Depression Therapy System | fdanews.com 20. A Tool For The Mind | Marom Bikson | TEDxBushwick on youtube 21. TDCS for Cognitive Enhancement talk by Vince Clark on youtube 22. Trumbo, M., Matzen, L., Coffman, B., Hunter, M., Jones, A., Robinson, C. and Clark, V., 2020. Enhanced Working Memory Performance Via Transcranial Direct Current Stimulation: The Possibility Of Near And Far Transfer. 23. Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, Cohen LG, Fregni F, Herrmann CS, Kappenman ES, Knotkova H, Liebetanz D, Miniussi C, Miranda PC, Paulus W, Priori A, Reato D, Stagg C, Wenderoth N, Nitsche MA. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. 2016 Feb;127(2):1031-48